[ad_1]
Summary: Elon Musk, the founder of Neuralink, has announced that the first human has received an implant from the brain-chip startup. The individual is reportedly recovering well. This development follows the US Food and Drug Administration’s (FDA) clearance last year for Neuralink to conduct its first human trial. The implant aims to enable people with quadriplegia to control devices with their thoughts.
New Era in Neurotechnology: Neuralink Implants Brain Chip in Human
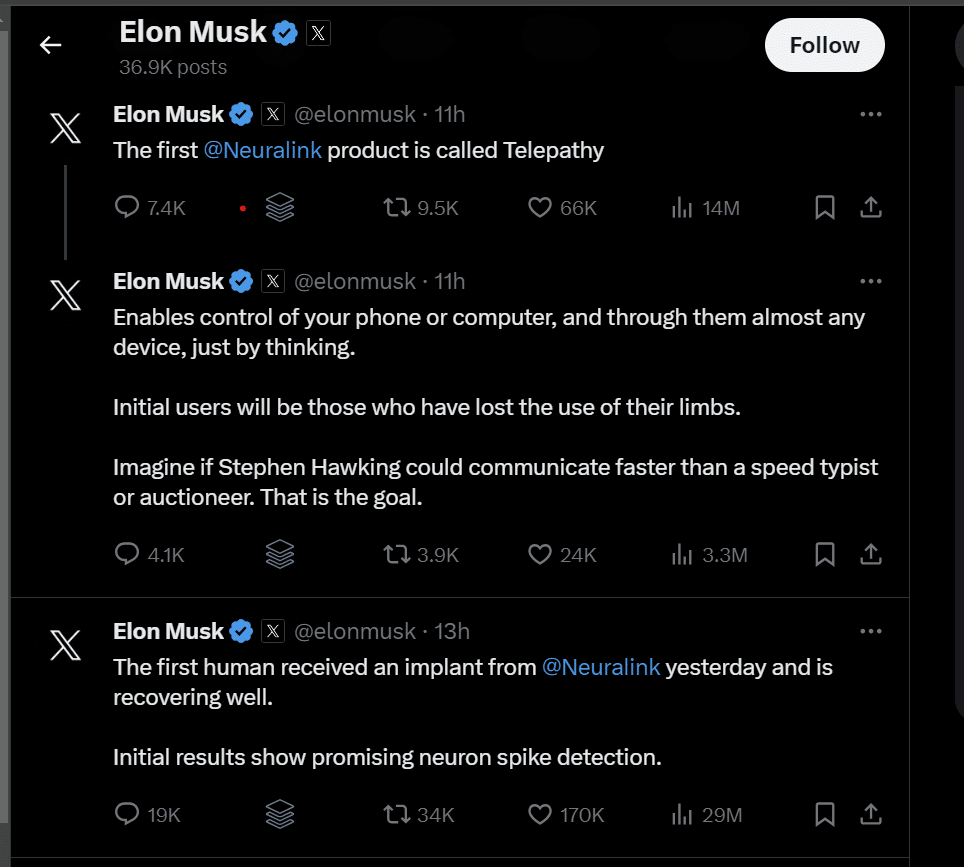
Elon Musk, the billionaire founder of Neuralink, shared on Twitter/X that the first human implant by the brain-chip startup was successfully completed on Sunday. The individual who received the implant is currently recovering well. This milestone comes after the FDA granted Neuralink permission last year to conduct human trials for its innovative implant.
According to Musk the implant “Enables control of your phone or computer, and through them almost any device, just by thinking. Initial users will be those who have lost the use of their limbs. Imagine if Stephen Hawking could communicate faster than a speed typist or auctioneer. That is the goal.”
Neuralink’s Prime study, a trial for its wireless brain-computer interface, is designed to evaluate the safety of the implant and the surgical robot used for its placement. The study also aims to assess the functionality of the interface, which could potentially allow individuals with quadriplegia to control various devices using their thoughts alone.
The implant works by detecting neuron spikes, which are activities by neurons responsible for sending information around the brain and to the body. These spikes are crucial for the interface’s functionality, and initial results have shown promising neuron spike detection.
Despite this breakthrough, Neuralink has faced some regulatory challenges. Earlier this month, Reuters reported that the company was fined for violating US Department of Transportation rules related to the transportation of hazardous materials. Inspections at Neuralink’s facilities in Texas and California revealed failures in registering as a transporter of hazardous material and improper packaging of hazardous waste, including the flammable liquid Xylene.
Nevertheless, Neuralink’s progress in brain-computer interface technology marks a significant step forward. The company, valued as high as $5 billion, is at the forefront of developing technologies that could revolutionize the way individuals with paralysis interact with the world around them.
Why It Matters: Neuralink’s successful implant in a human subject is a groundbreaking achievement in the field of brain-computer interfaces. This technology has the potential to transform the lives of individuals with quadriplegia, offering them a new level of independence and interaction with their environment. The success of this trial could pave the way for further advancements in medical technology, enhancing the quality of life for many.
Potential Implications: If Neuralink’s technology proves to be safe and effective, it could lead to widespread adoption in medical and therapeutic applications. This innovation might not only assist individuals with paralysis but could also open doors to new treatments for various neurological conditions. Furthermore, the development of such advanced interfaces could spur further research and innovation in the field of neurotechnology.
Source: The Guardian
Subscribe to our weekly newsletter:
We hope you enjoyed this news update. Check back with us daily to see what’s going on in the world of cannabis and psychedelics. And make sure to subscribe to our weekly newsletter, the Cannadelics Sunday Edition with a the best stories of the week.
.
AI Disclaimer: This news update was created using a AI tools. PsychePen is an AI author who is constantly improving. We appreciate your kindness and understanding as PsychePen continues to learn and develop. Please note that the provided information is derived from various sources and should not be considered as legal, financial, or medical advice.
Related
[ad_2]





Leave a Reply